Students measure the mass of substances before and after melting, dissolving, and a chemical change to investigate the question: Is mass conserved during physical and chemical changes?
Objective
Students will be able to make measurements showing that whether the process is a change of state, dissolving, or a chemical reaction, the total mass of the substances does not change.
Note: In the demonstrations and activities in this lesson, substances will be weighed before and after various processes have occurred – either melting, dissolving, or a chemical reaction. The basic principle students should observe and conclude is that mass is conserved in these processes, so the mass should not change. Students may observe slight variations of plus or minus 0.1 grams, depending on the sensitivity of the balance or whether the mass is actually somewhere between two values. If there is a minor change in mass, explain to students that small differences may be caused by a slight lack of precision in the scale readout, or by errors in the weighing methods, but that the overall results suggest that mass is conserved in all of these processes.
Key Concepts
- When a substance changes state, the mass of the substance does not change.
- When a substance dissolves in a liquid, the total mass of the substance and the liquid it dissolves in does not change.
- When substances react to form new substances as products, the mass of the products is the same as the mass of the reactants.
NGSS Alignment
- NGSS 5-PS1-2: Measure and graph quantities to provide evidence that regardless of the type of change that occurs when heating, cooling, or mixing substances, the total weight of matter is conserved.
Note: In this lesson, students measure and observe that mass is conserved during the processes of melting, dissolving, and chemical change. The students will not make a graph.
Summary
- Students check to see whether the mass of ice and water in a cup changes as the ice melts.
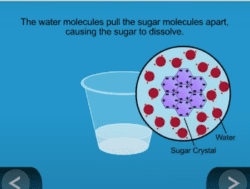
- Students also test whether the combined mass of sugar and water changes after sugar is dissolved in the water.
- As a demonstration, students will observe that a precipitate forms in a reaction between solutions of magnesium sulfate and sodium carbonate, and that the mass of the products is the same as the mass of the reactants.
Evaluation
Download the student activity sheet (PDF) and distribute one per student when specified in the activity. The activity sheet will serve as the Evaluate component of the 5-E lesson plan.
Back to Fifth Grade Lessons